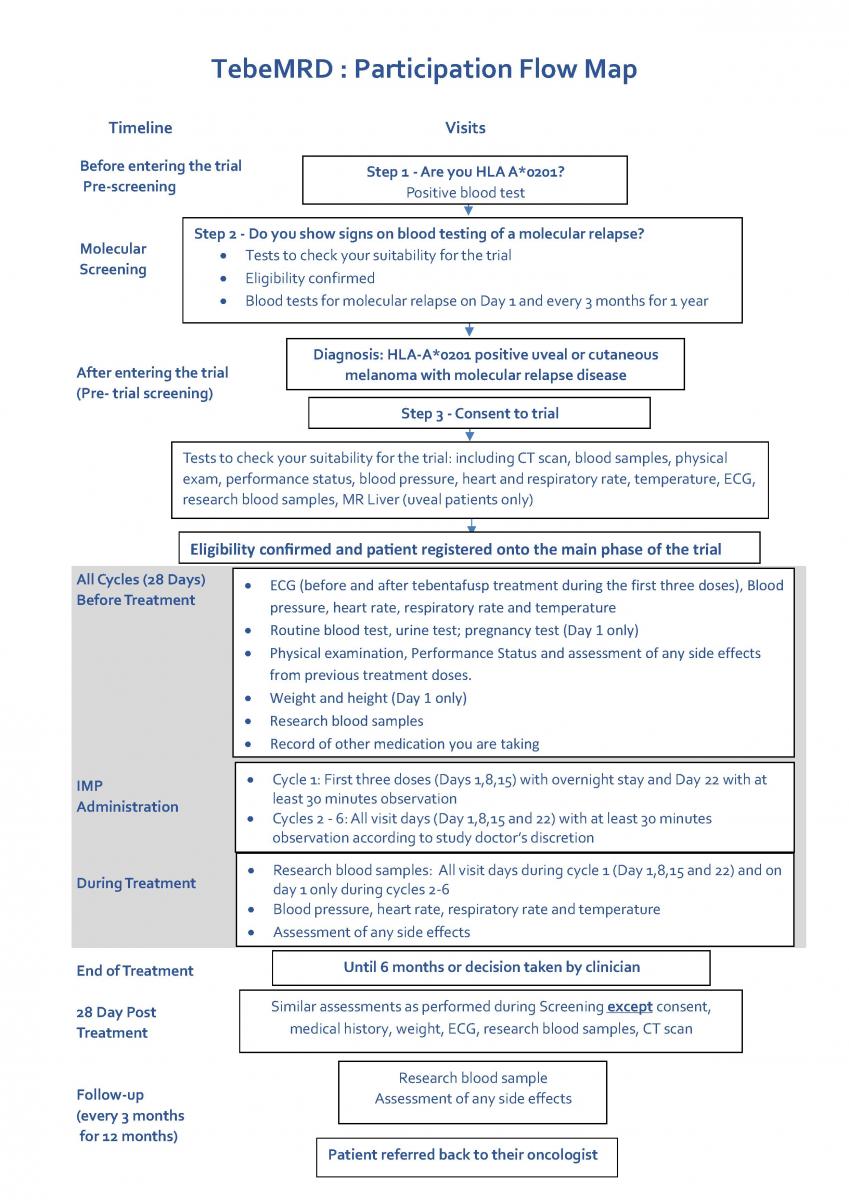
What would taking part involve? What are the three phases of the trial?
Pre-Screening:
This stage of the trial involves only a blood test but we will also talk you through what will happen in the later stages of the trial to help with your decision about taking part. If you decide that you would like to take part, we will ask you to sign a consent form. You will then have the blood sample taken for HLA-A*0201 testing. The blood sample is about 10ml, approximately 2 teaspoons. If a suitable blood sample has already been taken and is available, a fresh blood sample may not need to be taken. About half of the general population are HLA type A*0201 positive. Therefore, it is anticipated that roughly half of the patients who enter this pre-screening stage will be eligible for the molecular screening phase.
Molecular Screening:
This phase of the study involves up to 9 visits in total, over a 24-month period. Each visit should take approximately 30 minutes and wherever possible will be scheduled to combine with your standard care.
A research nurse will talk you through what will happen in the molecular screening phase of the trial. After you have had the opportunity to ask any questions you might have and if you decide that you would like to take part, will ask you to sign a consent form. You will then have various tests performed (detail on the exact tests can be found within the Molecular Screening - Patient Information Sheet (V5.0 23Feb2024).
We will also ask your permission to collect the sample of cancer (biopsy) that was taken when you were first diagnosed. We will use as little of this as we need, to compare the results of the blood tests for ctDNA to the DNA in your original tumour.
If during one of the visits your blood tests show traces of melanoma, you may become eligible to receive tebentafusp. If, however, after all 9 visits, you are shown to be negative to molecular relapse disease, you will not be able to continue into the main study.
Main Treatment Phase:
The main study consists of weekly visits for up to 6 months so potentially involving up to 24 clinic visits. Wherever possible these visits will be scheduled to combine with your standard care. The study drug, tebentafusp is given as an intravenous infusion (into your bloodstream) usually over about 15 to 20 minutes. You will be required to be admitted overnight following the first three doses of the study treatment. In addition to receiving tebentafusp you will receive all the other care that you would normally receive. If you develop serious side effects, or are unable to follow trial procedures, you may be withdrawn from the trial treatment.
You may be withdrawn from treatment early if your trial doctor establishes that the treatment is not benefitting you, if your tumour is progressive during the trial, if you voluntarily withdraw consent or if you do not attend for trial visits and we are unable to contact you. We will, however, ask you to consent for us to use any information we have already collected about the treatment in your case, up until the point you withdrew from the study.
After your last dose of tebentafusp, you will be asked to return to the hospital where you had treatment for follow-up visits every 3 months, +/- 10 days, for a year (4 visits in total). These visits should take approximately an hour depending on whether they involve scans etc. However, wherever possible these visits will be scheduled to combine with your standard care. During these follow up visits you will have blood tests for molecular relapse and a review of any side effects you are experiencing.
After your final trial visit (approximately 12 months after your final tebentafusp treatment), we will continue to contact your local hospital for an update on your health every 3 months, until the study comes to an end. We will not contact you directly, and you will not need to visit the hospital.
After your participation in the study ends, your care will be transferred back to the doctor who cared for you before you entered the trial.